This page was generated from
docs/Examples/Cpx_Cpx_Liq_Thermobarometry/Cpx_Liq_Thermobarometry.ipynb.
Interactive online version:
.
Clinopyroxene-only and Clinopyroxene-Liquid Thermobarometry.
This notebook goes through the options for clinopyroxene-Liquid thermobarometry and clinopyroxene-only thermobarometry
Cpx-Liq matching is not covered in this tutorial, there is a separate folder “Cpx_Liquid_melt_matching” for that
You can download the excel spreadsheet from: https://github.com/PennyWieser/Thermobar/blob/main/docs/Examples/Cpx_Cpx_Liq_Thermobarometry/Cpx_Liq_Example.xlsx
You need to install Thermobar once on your machine, if you haven’t done this yet, uncomment the line below (remove the #)
[1]:
#!pip install Thermobar
First, load the necessary python things
[2]:
import numpy as np
import pandas as pd
import matplotlib.pyplot as plt
import Thermobar as pt
Now, load the data
[3]:
out=pt.import_excel('Cpx_Liq_Example.xlsx', sheet_name="Sheet1")
my_input=out['my_input']
Liqs=out['Liqs']
Cpxs=out['Cpxs']
Inspect the data to check it loaded properly
[4]:
Liqs.head()
[4]:
| SiO2_Liq | TiO2_Liq | Al2O3_Liq | FeOt_Liq | MnO_Liq | MgO_Liq | CaO_Liq | Na2O_Liq | K2O_Liq | Cr2O3_Liq | P2O5_Liq | H2O_Liq | Fe3Fet_Liq | NiO_Liq | CoO_Liq | CO2_Liq | Sample_ID_Liq | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 51.1 | 0.93 | 17.5 | 8.91 | 0.18 | 6.09 | 11.50 | 3.53 | 0.17 | 0.0 | 0.15 | 3.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0 |
| 1 | 51.5 | 1.19 | 19.2 | 8.70 | 0.19 | 4.98 | 10.00 | 3.72 | 0.42 | 0.0 | 0.14 | 6.2 | 0.0 | 0.0 | 0.0 | 0.0 | 1 |
| 2 | 59.1 | 0.54 | 19.1 | 5.22 | 0.19 | 3.25 | 7.45 | 4.00 | 0.88 | 0.0 | 0.31 | 6.2 | 0.0 | 0.0 | 0.0 | 0.0 | 2 |
| 3 | 52.5 | 0.98 | 19.2 | 8.04 | 0.20 | 4.99 | 9.64 | 4.15 | 0.21 | 0.0 | 0.14 | 6.2 | 0.0 | 0.0 | 0.0 | 0.0 | 3 |
| 4 | 56.2 | 0.34 | 20.4 | 5.88 | 0.20 | 2.58 | 7.18 | 6.02 | 1.02 | 0.0 | 0.23 | 6.2 | 0.0 | 0.0 | 0.0 | 0.0 | 4 |
[5]:
Cpxs.head()
[5]:
| SiO2_Cpx | TiO2_Cpx | Al2O3_Cpx | FeOt_Cpx | MnO_Cpx | MgO_Cpx | CaO_Cpx | Na2O_Cpx | K2O_Cpx | Cr2O3_Cpx | Sample_ID_Cpx | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 51.5 | 0.50 | 3.70 | 5.18 | 0.09 | 15.8 | 22.8 | 0.24 | 0.0 | 0.66 | 0 |
| 1 | 50.3 | 0.73 | 4.12 | 5.83 | 0.00 | 15.0 | 22.7 | 0.24 | 0.0 | 0.28 | 1 |
| 2 | 47.3 | 1.75 | 7.85 | 6.51 | 0.14 | 13.1 | 22.5 | 0.25 | 0.0 | 0.22 | 2 |
| 3 | 51.1 | 0.63 | 4.41 | 5.66 | 0.13 | 15.6 | 22.6 | 0.23 | 0.0 | 0.27 | 3 |
| 4 | 51.0 | 0.56 | 4.14 | 7.33 | 0.20 | 14.4 | 22.4 | 0.31 | 0.0 | 0.09 | 4 |
Getting help
At any point, you can do help(pt.function) to get some more information
For example, here we get information on inputs/outputs for Cpx-Liq thermometry, including the equation options
[6]:
help(pt.calculate_cpx_liq_temp)
Help on function calculate_cpx_liq_temp in module Thermobar.clinopyroxene_thermobarometry:
calculate_cpx_liq_temp(*, equationT, cpx_comps=None, liq_comps=None, meltmatch=None, P=None, eq_tests=False, H2O_Liq=None, Fe3Fet_Liq=None, sigma=1, Kd_Err=0.03)
Clinopyroxene-Liquid thermometry, calculates temperature in Kelvin
(and equilibrium tests as an option)
Parameters
-------
cpx_comps: pandas.DataFrame
Clinopyroxene compositions with column headings SiO2_Cpx, MgO_Cpx etc.
liq_comps: pandas.DataFrame
Liquid compositions with column headings SiO2_Liq, MgO_Liq etc.
Or:
meltmatch: pandas.DataFrame
Combined dataframe of cpx-Liquid compositions
Used for calculate_cpx_liq_press_temp_matching function.
EquationT: str
Choice of equation:
Cpx-Liquid
| T_Put1996_eqT1 (P-indep, H2O-indep)
| T_Mas2013_eqTalk1 (P-indep, H2O-indep, alk adaption of T1)
| T_Brug2019 (P-indep, H2O-indep)
| T_Put1996_eqT2 (P-dep, H2O-indep)
| T_Mas2013_eqTalk2 (P-dep, H2O-indep, alk adaption of T2)
| T_Put1999 (P-dep, H2O-indep)
| T_Put2003 (P-dep, H2O-indep)
| T_Put1999 (P-dep, H2O-indep)
| T_Put2008_eq33 (P-dep, H2O-dep)
| T_Mas2013_eqalk33 (P-dep, H2O-dep, alk adaption of eq33)
| T_Mas2013_Palk2012 (P-indep, H2O_dep)
| T_Petrelli2020_Cpx_Liq (gives voting)
| T_Jorgenson2022_Cpx_Liq (gives voting)
| T_Petrelli2020_Cpx_Liq_onnx (gives consistent result every time)
P: float, int, pandas.Series, str ("Solve")
Pressure in kbar
Only needed for P-sensitive thermometers.
If enter P="Solve", returns a partial function
Else, enter an integer, float, or panda series
eq_tests: bool
If False, just returns pressure (default) as a panda series
If True, returns pressure, Values of Eq tests,
Values of Eq tests (Kd, EnFs, DiHd, CaTs, CrCaTs),
as well as user-entered cpx and liq comps and components.
Returns
-------
If eq_tests=False
pandas.Series: Temperature in Kelvin
If eq_tests=True
pandas.DataFrame: Temperature in Kelvin +
Eq Tests + cpx+liq comps + components
Example 1 - Calculating Temperature
1a - Temperature for a known pressure and water content
Here, we calculate temperature using the H2O content given in the H2O_Liq column in the user-entered spreadsheet (the default), and P=5 kbar
There are a number of equations (see help above), but here we use T_Put2008_eq33 for temperature
[7]:
Temp_T33=pt.calculate_cpx_liq_temp(cpx_comps=Cpxs, liq_comps=Liqs,
equationT="T_Put2008_eq33", P=5)-273.15 # Default Kelvin
Temp_T33
[7]:
0 1091.562867
1 1031.605285
2 999.303442
3 1032.536881
4 979.726983
5 1076.821062
6 1096.927022
7 1097.227100
8 1113.361468
9 1084.298534
10 1091.178760
11 1071.691587
12 1089.996302
13 1028.359107
14 1028.359107
15 1028.359107
16 1093.611526
17 NaN
18 1087.546061
19 1104.700975
dtype: float64
1b - Temperature, overwriting the spreadsheet water content in the function itself
Here, we are reseting water to 0 wt%. You can see the temperatures are much higher
[8]:
Temp_T33_0H2O=pt.calculate_cpx_liq_temp(cpx_comps=Cpxs, liq_comps=Liqs,
equationT="T_Put2008_eq33",
P=5, H2O_Liq=0)-273.15
Temp_T33_0H2O
[8]:
0 1142.969951
1 1109.923744
2 1073.681511
3 1110.970579
4 1051.769286
5 1134.212162
6 1149.012230
7 1159.843300
8 1174.122141
9 1141.430504
10 1144.660690
11 1131.557607
12 1144.720092
13 1076.010017
14 1076.010017
15 1076.010017
16 1150.716488
17 NaN
18 1150.219143
19 1159.165100
dtype: float64
1c- Lets use the thermometer of Brugman and Till, 2019.
This returns a number of warnings, because the authors recomend a compositional calibration range, which our entered cpx compositions lie outside of.
[9]:
Temp_TBrug=pt.calculate_cpx_liq_temp(cpx_comps=Cpxs, liq_comps=Liqs,
equationT="T_Brug2019", P=5)-273.15
Temp_TBrug
Youve selected a P-independent function
C:\Users\penny\AppData\Local\Temp\ipykernel_23648\1899851194.py:1: UserWarning: Some inputted CPX compositions have Cpx Mg#>0.65;.
Temp_TBrug=pt.calculate_cpx_liq_temp(cpx_comps=Cpxs, liq_comps=Liqs,
C:\Users\penny\AppData\Local\Temp\ipykernel_23648\1899851194.py:1: UserWarning: Some inputted CPX compositions have Al2O3>7 wt%;.
Temp_TBrug=pt.calculate_cpx_liq_temp(cpx_comps=Cpxs, liq_comps=Liqs,
C:\Users\penny\AppData\Local\Temp\ipykernel_23648\1899851194.py:1: UserWarning: Some inputted Liq compositions have SiO2<70 wt%;
Temp_TBrug=pt.calculate_cpx_liq_temp(cpx_comps=Cpxs, liq_comps=Liqs,
c:\users\penny\box\postdoc\mybarometers\thermobar_outer\src\Thermobar\clinopyroxene_thermobarometry.py:2213: UserWarning: which is outside the recomended calibration range of Brugman and Till (2019)
w.warn("which is outside the recomended calibration range of Brugman and Till (2019)")
[9]:
0 1797.689417
1 1632.682392
2 1202.363348
3 1558.356437
4 1028.947694
5 1828.840169
6 1889.290499
7 1772.472552
8 1946.324399
9 1873.382098
10 1881.239956
11 1834.031996
12 1853.418730
13 1868.159678
14 1868.159678
15 1868.159678
16 1836.411331
17 1857.905516
18 1928.817708
19 1973.023872
dtype: float64
1d - We can also specify eq_tests=True to get a full dataframe back with all the components, as well as a number of equilibrium test values
You could then extract just the temps using Temp_T33_0H2O_EqTests[‘T_K_calc’] or any other column you want the same way
[10]:
Temp_T33_0H2O_EqTests=pt.calculate_cpx_liq_temp(cpx_comps=Cpxs, liq_comps=Liqs,
equationT="T_Put2008_eq33",
P=5, H2O_Liq=0, eq_tests=True)
Temp_T33_0H2O_EqTests
Using Fe3FeT from input file to calculate Kd Fe-Mg
[10]:
| P_kbar_calc | T_K_calc | Eq Tests Neave2017? | Delta_Kd_Put2008 | Delta_Kd_Mas2013 | Delta_EnFs_Mollo13 | Delta_EnFs_Put1999 | Delta_CaTs_Put1999 | Delta_DiHd_Mollo13 | Delta_DiHd_Put1999 | ... | Delta_EnFs_I_M_Mollo13 | CaTs_Pred_Put1999 | Delta_CaTs_I_M_Put1999 | CrCaTs_Pred_Put1999 | Delta_CrCaTs_I_M_Put1999 | CaTi_Pred_Put1999 | Delta_CaTi_I_M_Put1999 | Jd_Pred_Put1999 | Delta_Jd_Put1999 | Delta_Jd_I_M_Put1999 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 1416.119951 | False | 0.042816 | 0.094027 | 0.010858 | 0.021183 | 0.016957 | 0.089424 | 0.001586 | ... | -0.010858 | 0.013418 | -0.016957 | 0.000000 | 0.009562 | 0.043320 | 0.002307 | 0.016184 | 0.000872 | 0.000872 |
| 1 | 5 | 1383.073744 | False | 0.036792 | 0.090778 | 0.007575 | 0.021866 | 0.023796 | 0.104285 | 0.024207 | ... | -0.007575 | 0.013021 | -0.023796 | 0.000000 | 0.004122 | 0.056932 | 0.011994 | 0.017429 | 0.000099 | 0.000099 |
| 2 | 5 | 1346.831511 | False | 0.058659 | 0.172858 | 0.043395 | 0.013382 | 0.075254 | 0.006413 | 0.103915 | ... | 0.043395 | 0.016630 | -0.075254 | 0.000000 | 0.003245 | 0.029418 | 0.042249 | 0.018331 | 0.000246 | 0.000246 |
| 3 | 5 | 1384.120579 | False | 0.034331 | 0.101144 | 0.012488 | 0.028698 | 0.031526 | 0.084045 | 0.024697 | ... | -0.012488 | 0.014301 | -0.031526 | 0.000000 | 0.003909 | 0.050962 | 0.009764 | 0.019332 | 0.002997 | 0.002997 |
| 4 | 5 | 1324.919286 | False | 0.022156 | 0.097447 | 0.021106 | 0.049019 | 0.032121 | 0.071950 | 0.035481 | ... | -0.021106 | 0.010990 | -0.032121 | 0.000000 | 0.001315 | 0.050713 | 0.014761 | 0.027953 | 0.005738 | 0.005738 |
| 5 | 5 | 1407.362162 | False | 0.011745 | 0.133688 | 0.008645 | 0.029286 | 0.012999 | 0.062059 | 0.001463 | ... | -0.008645 | 0.012999 | 0.012999 | 0.297720 | 0.277125 | 0.057369 | 0.026926 | 0.019470 | 0.006126 | 0.006126 |
| 6 | 5 | 1422.162230 | False | 0.011959 | 0.091270 | 0.008903 | 0.024363 | 0.014640 | 0.070686 | 0.003636 | ... | -0.008903 | 0.014640 | 0.014640 | 0.130559 | 0.110135 | 0.052890 | 0.032902 | 0.015664 | 0.002722 | 0.002722 |
| 7 | 5 | 1432.993300 | False | 0.052296 | 0.077646 | 0.007652 | 0.024590 | 0.015888 | 0.054068 | 0.010131 | ... | -0.007652 | 0.015888 | 0.015888 | 0.148010 | 0.125617 | 0.045423 | 0.029060 | 0.018272 | 0.011840 | 0.011840 |
| 8 | 5 | 1447.272141 | False | 0.046595 | 0.085580 | 0.020659 | 0.043637 | 0.007563 | 0.082456 | 0.039311 | ... | -0.020659 | 0.016662 | 0.007563 | 0.001101 | 0.017485 | 0.052715 | 0.016339 | 0.015821 | 0.008622 | 0.008622 |
| 9 | 5 | 1414.580504 | False | 0.018097 | 0.116199 | 0.015372 | 0.032515 | 0.013343 | 0.083869 | 0.008159 | ... | -0.015372 | 0.013343 | 0.013343 | 0.003516 | 0.010965 | 0.059753 | 0.014958 | 0.018210 | 0.004591 | 0.004591 |
| 10 | 5 | 1417.810690 | True | 0.015009 | 0.141280 | 0.004910 | 0.020402 | 0.014748 | 0.058127 | 0.012136 | ... | -0.004910 | 0.014748 | 0.014748 | 0.857156 | 0.836370 | 0.043707 | 0.041545 | 0.016472 | 0.003230 | 0.003230 |
| 11 | 5 | 1404.707607 | True | 0.015558 | 0.109274 | 0.013261 | 0.021390 | 0.015494 | 0.050272 | 0.041881 | ... | -0.013261 | 0.015494 | 0.015494 | 0.001648 | 0.014651 | 0.047981 | 0.032592 | 0.017360 | 0.009224 | 0.009224 |
| 12 | 5 | 1417.870092 | False | 0.044544 | 0.063790 | 0.019190 | 0.035825 | 0.014555 | 0.060011 | 0.001347 | ... | -0.019190 | 0.014555 | 0.014555 | 0.574581 | 0.555774 | 0.052013 | 0.036050 | 0.016889 | 0.002411 | 0.002411 |
| 13 | 5 | 1349.160017 | True | 0.014011 | 0.133748 | 0.047904 | 0.041739 | 0.012404 | 0.016132 | 0.150795 | ... | -0.047904 | 0.012404 | 0.012404 | 0.052732 | 0.042651 | 0.054363 | 0.026279 | 0.019044 | 0.018319 | 0.018319 |
| 14 | 5 | 1349.160017 | True | 0.014011 | 0.133748 | 0.047904 | 0.041739 | 0.012404 | 0.016132 | 0.150795 | ... | -0.047904 | 0.012404 | 0.012404 | 0.052732 | 0.042651 | 0.054363 | 0.026279 | 0.019044 | 0.018319 | 0.018319 |
| 15 | 5 | 1349.160017 | True | 0.014011 | 0.133748 | 0.047904 | 0.041739 | 0.012404 | 0.016132 | 0.150795 | ... | -0.047904 | 0.012404 | 0.012404 | 0.052732 | 0.042651 | 0.054363 | 0.026279 | 0.019044 | 0.018319 | 0.018319 |
| 16 | 5 | 1423.866488 | True | 0.002518 | 0.111409 | 0.013153 | 0.031786 | 0.015716 | 0.050165 | 0.001366 | ... | -0.013153 | 0.015716 | 0.015716 | 0.356916 | 0.342040 | 0.052646 | 0.035059 | 0.018291 | 0.002126 | 0.002126 |
| 17 | 5 | NaN | False | NaN | NaN | NaN | NaN | NaN | NaN | NaN | ... | NaN | NaN | NaN | 0.603117 | 0.588110 | NaN | NaN | NaN | NaN | NaN |
| 18 | 5 | 1423.369143 | False | 0.006558 | 0.108254 | 0.012578 | 0.032440 | 0.014620 | 0.067687 | 0.009856 | ... | -0.012578 | 0.014620 | 0.014620 | 0.160617 | 0.141226 | 0.060201 | 0.021519 | 0.017590 | 0.003868 | 0.003868 |
| 19 | 5 | 1432.315100 | False | 0.007279 | 0.123765 | 0.011986 | 0.036870 | 0.014680 | 0.077193 | 0.026064 | ... | -0.011986 | 0.014680 | 0.014680 | 0.310037 | 0.296888 | 0.056968 | 0.030100 | 0.016562 | 0.002476 | 0.002476 |
20 rows × 130 columns
Example 2 - Calculating pressure for a known temperature
2a - Pressure at fixed temperature (T=1300 K), and pressures from Neave and Putirka (2017)
[11]:
P_FixedTNeave=pt.calculate_cpx_liq_press(cpx_comps=Cpxs, liq_comps=Liqs,
equationP="P_Neave2017", T=1300)
P_FixedTNeave
[11]:
0 0.634602
1 1.655874
2 1.146083
3 1.028349
4 3.854147
5 0.077818
6 0.103202
7 3.902001
8 3.198764
9 -0.172497
10 -0.067569
11 -2.437195
12 0.531826
13 -14.887115
14 -14.887115
15 -14.887115
16 1.346623
17 NaN
18 0.405712
19 1.682571
dtype: float64
2b - Equation 30 from Putirka (2008), overwriting input water, return equilibrium tests
Here we change equation P, overwrite the H2O content in the function, and ask for equilibrium tests.
We are selecting equation 30 from Putirka (2008) this time, T=1300 K
[12]:
Temp_P30_0H2O=pt.calculate_cpx_liq_press(cpx_comps=Cpxs, liq_comps=Liqs,
equationP="P_Put2008_eq30",
T=1300, H2O_Liq=0, eq_tests=True)
Temp_P30_0H2O
Using Fe3FeT from input file to calculate Kd Fe-Mg
[12]:
| P_kbar_calc | T_K_calc | Eq Tests Neave2017? | Delta_Kd_Put2008 | Delta_Kd_Mas2013 | Delta_EnFs_Mollo13 | Delta_EnFs_Put1999 | Delta_CaTs_Put1999 | Delta_DiHd_Mollo13 | Delta_DiHd_Put1999 | ... | Delta_EnFs_I_M_Mollo13 | CaTs_Pred_Put1999 | Delta_CaTs_I_M_Put1999 | CrCaTs_Pred_Put1999 | Delta_CrCaTs_I_M_Put1999 | CaTi_Pred_Put1999 | Delta_CaTi_I_M_Put1999 | Jd_Pred_Put1999 | Delta_Jd_Put1999 | Delta_Jd_I_M_Put1999 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | -2.090909 | 1300 | False | 0.015390 | 0.116829 | 0.002696 | 0.003912 | 0.019173 | 0.089851 | 0.350659 | ... | -0.002696 | 0.011202 | -0.019173 | 0.000000 | 0.009562 | 0.047180 | 0.006167 | 0.016165 | 0.000891 | 0.000891 |
| 1 | -2.604072 | 1300 | True | 0.016996 | 0.108121 | 0.006605 | 0.005373 | 0.025431 | 0.049622 | 0.286210 | ... | 0.006605 | 0.011385 | -0.025431 | 0.000000 | 0.004122 | 0.060657 | 0.015718 | 0.017407 | 0.000077 | 0.000077 |
| 2 | -2.145841 | 1300 | False | 0.069927 | 0.183575 | 0.074576 | 0.029940 | 0.076260 | 0.125978 | 0.249574 | ... | 0.074576 | 0.015625 | -0.076260 | 0.000000 | 0.003245 | 0.030292 | 0.041375 | 0.018308 | 0.000223 | 0.000223 |
| 3 | -2.334004 | 1300 | False | 0.014291 | 0.117657 | 0.003134 | 0.008701 | 0.033245 | 0.066274 | 0.281808 | ... | 0.003134 | 0.012582 | -0.033245 | 0.000000 | 0.003909 | 0.054136 | 0.012939 | 0.019308 | 0.002973 | 0.002973 |
| 4 | -0.177987 | 1300 | False | 0.016126 | 0.102892 | 0.007476 | 0.045035 | 0.032562 | 0.004383 | 0.113023 | ... | -0.007476 | 0.010549 | -0.032562 | 0.000000 | 0.001315 | 0.051697 | 0.015744 | 0.027927 | 0.005712 | 0.005712 |
| 5 | -3.656248 | 1300 | False | 0.037163 | 0.157156 | 0.008999 | 0.005334 | 0.011085 | 0.129807 | 0.319319 | ... | 0.008999 | 0.011085 | 0.011085 | 0.297720 | 0.277125 | 0.061850 | 0.022445 | 0.019441 | 0.006098 | 0.006098 |
| 6 | -3.959117 | 1300 | False | 0.016846 | 0.121449 | 0.007682 | 0.004097 | 0.012259 | 0.135041 | 0.353721 | ... | 0.007682 | 0.012259 | 0.012259 | 0.130559 | 0.110135 | 0.057515 | 0.028277 | 0.015640 | 0.002698 | 0.002698 |
| 7 | 0.216684 | 1300 | False | 0.021028 | 0.105244 | 0.007877 | 0.010227 | 0.013249 | 0.108774 | 0.364703 | ... | -0.007877 | 0.013249 | 0.013249 | 0.148010 | 0.125617 | 0.049502 | 0.024980 | 0.018258 | 0.011854 | 0.011854 |
| 8 | -0.678349 | 1300 | False | 0.012104 | 0.115746 | 0.019128 | 0.005116 | 0.004551 | 0.094621 | 0.357156 | ... | -0.019128 | 0.013650 | 0.004551 | 0.001101 | 0.017485 | 0.057933 | 0.011121 | 0.015807 | 0.008636 | 0.008636 |
| 9 | -3.118628 | 1300 | False | 0.008977 | 0.139129 | 0.002217 | 0.008054 | 0.011220 | 0.104703 | 0.331041 | ... | -0.002217 | 0.011220 | 0.011220 | 0.003516 | 0.010965 | 0.064853 | 0.009859 | 0.018185 | 0.004566 | 0.004566 |
| 10 | -3.748095 | 1300 | False | 0.042821 | 0.166301 | 0.012878 | 0.009837 | 0.012445 | 0.143123 | 0.361588 | ... | 0.012878 | 0.012445 | 0.012445 | 0.857156 | 0.836370 | 0.047356 | 0.037895 | 0.016447 | 0.003205 | 0.003205 |
| 11 | -5.694960 | 1300 | False | 0.009248 | 0.131688 | 0.014536 | 0.006415 | 0.013332 | 0.163945 | 0.361704 | ... | 0.014536 | 0.013332 | 0.013332 | 0.001648 | 0.014651 | 0.051503 | 0.029071 | 0.017328 | 0.009192 | 0.009192 |
| 12 | -3.317520 | 1300 | False | 0.016718 | 0.092009 | 0.002553 | 0.004621 | 0.012336 | 0.131747 | 0.336589 | ... | -0.002553 | 0.012336 | 0.012336 | 0.574581 | 0.555774 | 0.056241 | 0.031822 | 0.016866 | 0.002388 | 0.002388 |
| 13 | -15.728385 | 1300 | False | 0.025832 | 0.144548 | 0.038085 | 0.028394 | 0.011485 | 0.292357 | 0.321371 | ... | 0.038085 | 0.011485 | 0.011485 | 0.052732 | 0.042651 | 0.056334 | 0.024309 | 0.018975 | 0.018250 | 0.018250 |
| 14 | -15.728385 | 1300 | False | 0.025832 | 0.144548 | 0.038085 | 0.028394 | 0.011485 | 0.292357 | 0.321371 | ... | 0.038085 | 0.011485 | 0.011485 | 0.052732 | 0.042651 | 0.056334 | 0.024309 | 0.018975 | 0.018250 | 0.018250 |
| 15 | -15.728385 | 1300 | False | 0.025832 | 0.144548 | 0.038085 | 0.028394 | 0.011485 | 0.292357 | 0.321371 | ... | 0.038085 | 0.011485 | 0.011485 | 0.052732 | 0.042651 | 0.056334 | 0.024309 | 0.018975 | 0.018250 | 0.018250 |
| 16 | -2.753658 | 1300 | False | 0.026676 | 0.140028 | 0.000281 | 0.000271 | 0.013269 | 0.140658 | 0.350210 | ... | 0.000281 | 0.013269 | 0.013269 | 0.356916 | 0.342040 | 0.057028 | 0.030677 | 0.018268 | 0.002102 | 0.002102 |
| 17 | NaN | 1300 | False | 0.000180 | 0.108710 | NaN | 0.014766 | NaN | NaN | 0.316925 | ... | NaN | NaN | NaN | 0.603117 | 0.588110 | 0.058863 | 0.028817 | NaN | NaN | NaN |
| 18 | -3.583424 | 1300 | False | 0.022523 | 0.136594 | 0.003481 | 0.002846 | 0.012271 | 0.132330 | 0.343415 | ... | 0.003481 | 0.012271 | 0.012271 | 0.160617 | 0.141226 | 0.065393 | 0.016327 | 0.017565 | 0.003843 | 0.003843 |
| 19 | -1.870615 | 1300 | False | 0.023835 | 0.151004 | 0.003511 | 0.002818 | 0.012196 | 0.105839 | 0.342393 | ... | -0.003511 | 0.012196 | 0.012196 | 0.310037 | 0.296888 | 0.062186 | 0.024882 | 0.016543 | 0.002495 | 0.002495 |
20 rows × 130 columns
2c - As above, but setting a fixed Fe3Fet_Liq ratio
Can overwrite the Fe3Fet in the input spreadsheet to a different value, affects calculations of delta Kd as this uses just Fe2+ in the melt
Note, it is debated whether Kd Fe-Mg should be calculated with all Fe (to do that here, specify Fe3Fet_Liq=0, Putirka), or using just Fe2+ (e.g., Neave and Putirka, 2017)
you can compare the delta Kd Put 2008 in this option from the answers above. You can see, by adding 30% Fe3+, you have become further from equilibrium
[13]:
Temp_P30_0H2O_30Fe=pt.calculate_cpx_liq_press(cpx_comps=Cpxs,
liq_comps=Liqs, equationP="P_Put2008_eq30",
T=1300, H2O_Liq=0, Fe3Fet_Liq=0.3, eq_tests=True)
Temp_P30_0H2O_30Fe
[13]:
| P_kbar_calc | T_K_calc | Eq Tests Neave2017? | Delta_Kd_Put2008 | Delta_Kd_Mas2013 | Delta_EnFs_Mollo13 | Delta_EnFs_Put1999 | Delta_CaTs_Put1999 | Delta_DiHd_Mollo13 | Delta_DiHd_Put1999 | ... | Delta_EnFs_I_M_Mollo13 | CaTs_Pred_Put1999 | Delta_CaTs_I_M_Put1999 | CrCaTs_Pred_Put1999 | Delta_CrCaTs_I_M_Put1999 | CaTi_Pred_Put1999 | Delta_CaTi_I_M_Put1999 | Jd_Pred_Put1999 | Delta_Jd_Put1999 | Delta_Jd_I_M_Put1999 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | -2.090909 | 1300 | False | 0.080646 | 0.212865 | 0.002696 | 0.003912 | 0.019173 | 0.089851 | 0.350659 | ... | -0.002696 | 0.011202 | -0.019173 | 0.000000 | 0.009562 | 0.047180 | 0.006167 | 0.016165 | 0.000891 | 0.000891 |
| 1 | -2.604072 | 1300 | False | 0.078351 | 0.203468 | 0.006605 | 0.005373 | 0.025431 | 0.049622 | 0.286210 | ... | 0.006605 | 0.011385 | -0.025431 | 0.000000 | 0.004122 | 0.060657 | 0.015718 | 0.017407 | 0.000077 | 0.000077 |
| 2 | -2.145841 | 1300 | False | 0.202528 | 0.316176 | 0.074576 | 0.029940 | 0.076260 | 0.125978 | 0.249574 | ... | 0.074576 | 0.015625 | -0.076260 | 0.000000 | 0.003245 | 0.030292 | 0.041375 | 0.018308 | 0.000223 | 0.000223 |
| 3 | -2.334004 | 1300 | False | 0.082216 | 0.214165 | 0.003134 | 0.008701 | 0.033245 | 0.066274 | 0.281808 | ... | 0.003134 | 0.012582 | -0.033245 | 0.000000 | 0.003909 | 0.054136 | 0.012939 | 0.019308 | 0.002973 | 0.002973 |
| 4 | -0.177987 | 1300 | False | 0.079595 | 0.198613 | 0.007476 | 0.045035 | 0.032562 | 0.004383 | 0.113023 | ... | -0.007476 | 0.010549 | -0.032562 | 0.000000 | 0.001315 | 0.051697 | 0.015744 | 0.027927 | 0.005712 | 0.005712 |
| 5 | -3.656248 | 1300 | False | 0.155721 | 0.275715 | 0.008999 | 0.005334 | 0.011085 | 0.129807 | 0.319319 | ... | 0.008999 | 0.011085 | 0.011085 | 0.297720 | 0.277125 | 0.061850 | 0.022445 | 0.019441 | 0.006098 | 0.006098 |
| 6 | -3.959117 | 1300 | False | 0.126698 | 0.231301 | 0.007682 | 0.004097 | 0.012259 | 0.135041 | 0.353721 | ... | 0.007682 | 0.012259 | 0.012259 | 0.130559 | 0.110135 | 0.057515 | 0.028277 | 0.015640 | 0.002698 | 0.002698 |
| 7 | 0.216684 | 1300 | False | 0.072592 | 0.198864 | 0.007877 | 0.010227 | 0.013249 | 0.108774 | 0.364703 | ... | -0.007877 | 0.013249 | 0.013249 | 0.148010 | 0.125617 | 0.049502 | 0.024980 | 0.018258 | 0.011854 | 0.011854 |
| 8 | -0.678349 | 1300 | False | 0.085341 | 0.213191 | 0.019128 | 0.005116 | 0.004551 | 0.094621 | 0.357156 | ... | -0.019128 | 0.013650 | 0.004551 | 0.001101 | 0.017485 | 0.057933 | 0.011121 | 0.015807 | 0.008636 | 0.008636 |
| 9 | -3.118628 | 1300 | False | 0.115456 | 0.245608 | 0.002217 | 0.008054 | 0.011220 | 0.104703 | 0.331041 | ... | -0.002217 | 0.011220 | 0.011220 | 0.003516 | 0.010965 | 0.064853 | 0.009859 | 0.018185 | 0.004566 | 0.004566 |
| 10 | -3.748095 | 1300 | False | 0.163805 | 0.287285 | 0.012878 | 0.009837 | 0.012445 | 0.143123 | 0.361588 | ... | 0.012878 | 0.012445 | 0.012445 | 0.857156 | 0.836370 | 0.047356 | 0.037895 | 0.016447 | 0.003205 | 0.003205 |
| 11 | -5.694960 | 1300 | False | 0.115844 | 0.238283 | 0.014536 | 0.006415 | 0.013332 | 0.163945 | 0.361704 | ... | 0.014536 | 0.013332 | 0.013332 | 0.001648 | 0.014651 | 0.051503 | 0.029071 | 0.017328 | 0.009192 | 0.009192 |
| 12 | -3.317520 | 1300 | False | 0.078749 | 0.187476 | 0.002553 | 0.004621 | 0.012336 | 0.131747 | 0.336589 | ... | -0.002553 | 0.012336 | 0.012336 | 0.574581 | 0.555774 | 0.056241 | 0.031822 | 0.016866 | 0.002388 | 0.002388 |
| 13 | -15.728385 | 1300 | False | 0.139535 | 0.258251 | 0.038085 | 0.028394 | 0.011485 | 0.292357 | 0.321371 | ... | 0.038085 | 0.011485 | 0.011485 | 0.052732 | 0.042651 | 0.056334 | 0.024309 | 0.018975 | 0.018250 | 0.018250 |
| 14 | -15.728385 | 1300 | False | 0.139535 | 0.258251 | 0.038085 | 0.028394 | 0.011485 | 0.292357 | 0.321371 | ... | 0.038085 | 0.011485 | 0.011485 | 0.052732 | 0.042651 | 0.056334 | 0.024309 | 0.018975 | 0.018250 | 0.018250 |
| 15 | -15.728385 | 1300 | False | 0.139535 | 0.258251 | 0.038085 | 0.028394 | 0.011485 | 0.292357 | 0.321371 | ... | 0.038085 | 0.011485 | 0.011485 | 0.052732 | 0.042651 | 0.056334 | 0.024309 | 0.018975 | 0.018250 | 0.018250 |
| 16 | -2.753658 | 1300 | False | 0.140741 | 0.254093 | 0.000281 | 0.000271 | 0.013269 | 0.140658 | 0.350210 | ... | 0.000281 | 0.013269 | 0.013269 | 0.356916 | 0.342040 | 0.057028 | 0.030677 | 0.018268 | 0.002102 | 0.002102 |
| 17 | NaN | 1300 | False | 0.102375 | 0.211265 | NaN | 0.014766 | NaN | NaN | 0.316925 | ... | NaN | NaN | NaN | 0.603117 | 0.588110 | 0.058863 | 0.028817 | NaN | NaN | NaN |
| 18 | -3.583424 | 1300 | False | 0.134808 | 0.248879 | 0.003481 | 0.002846 | 0.012271 | 0.132330 | 0.343415 | ... | 0.003481 | 0.012271 | 0.012271 | 0.160617 | 0.141226 | 0.065393 | 0.016327 | 0.017565 | 0.003843 | 0.003843 |
| 19 | -1.870615 | 1300 | False | 0.136682 | 0.263851 | 0.003511 | 0.002818 | 0.012196 | 0.105839 | 0.342393 | ... | -0.003511 | 0.012196 | 0.012196 | 0.310037 | 0.296888 | 0.062186 | 0.024882 | 0.016543 | 0.002495 | 0.002495 |
20 rows × 130 columns
Example 3 - Iterating pressure and temperature
In reality, unlesa you are an experimentalist, you rarely know one of pressure or temperature
In Keith Putirka’s spreadsheets, you can link up columns to iterate P and T towards a solution, this can be done here using the function calculate_cpx_liq_press_temp…
3a - Iterating equation 30 from Putirka (2008) for P, and equation 33 from Putirka (2008) for T
Without specifying anything else, you get a dataframe with columns for calculated pressure and temperature
[14]:
PT_iter_30_31=pt.calculate_cpx_liq_press_temp(cpx_comps=Cpxs, liq_comps=Liqs,
equationP="P_Put2008_eq30",
equationT="T_Put2008_eq33")
PT_iter_30_31
[14]:
| P_kbar_calc | T_K_calc | Delta_P_kbar_Iter | Delta_T_K_Iter | |
|---|---|---|---|---|
| 0 | 2.530914 | 1352.408784 | 0.0 | 0.0 |
| 1 | 1.786845 | 1290.151507 | 0.0 | 0.0 |
| 2 | 1.171520 | 1255.933868 | 0.0 | 0.0 |
| 3 | 2.143416 | 1292.669093 | 0.0 | 0.0 |
| 4 | 2.763538 | 1243.469600 | 0.0 | 0.0 |
| 5 | 0.535148 | 1328.353392 | 0.0 | 0.0 |
| 6 | 0.493360 | 1347.611001 | 0.0 | 0.0 |
| 7 | 6.377576 | 1377.397773 | 0.0 | 0.0 |
| 8 | 5.742168 | 1390.374437 | 0.0 | 0.0 |
| 9 | 1.396263 | 1339.752950 | 0.0 | 0.0 |
| 10 | 0.644651 | 1342.785453 | 0.0 | 0.0 |
| 11 | -1.883972 | 1312.045437 | 0.0 | 0.0 |
| 12 | 1.211110 | 1344.398677 | 0.0 | 0.0 |
| 13 | -14.685454 | 1217.300913 | 0.0 | 0.0 |
| 14 | -14.685454 | 1217.300913 | 0.0 | 0.0 |
| 15 | -14.685454 | 1217.300913 | 0.0 | 0.0 |
| 16 | 2.168816 | 1352.629530 | 0.0 | 0.0 |
| 17 | NaN | NaN | NaN | NaN |
| 18 | 1.315338 | 1342.522331 | 0.0 | 0.0 |
| 19 | 3.494361 | 1370.176239 | 0.0 | 0.0 |
3b - Same as above, but with eq_tests=True
Get all equilibrium tests, and input compostions as a larger dataframe.
[15]:
PT_iter_30_31_EqTests=pt.calculate_cpx_liq_press_temp(cpx_comps=Cpxs, liq_comps=Liqs, equationP="P_Put2008_eq30",
equationT="T_Put2008_eq33", eq_tests=True)
PT_iter_30_31_EqTests
Using Fe3FeT from input file to calculate Kd Fe-Mg
[15]:
| P_kbar_calc | T_K_calc | Eq Tests Neave2017? | Delta_P_kbar_Iter | Delta_T_K_Iter | Delta_Kd_Put2008 | Delta_Kd_Mas2013 | Delta_EnFs_Mollo13 | Delta_EnFs_Put1999 | Delta_CaTs_Put1999 | ... | Delta_EnFs_I_M_Mollo13 | CaTs_Pred_Put1999 | Delta_CaTs_I_M_Put1999 | CrCaTs_Pred_Put1999 | Delta_CrCaTs_I_M_Put1999 | CaTi_Pred_Put1999 | Delta_CaTi_I_M_Put1999 | Jd_Pred_Put1999 | Delta_Jd_Put1999 | Delta_Jd_I_M_Put1999 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.530914 | 1352.408784 | True | 0.0 | 0.0 | 0.027981 | 0.106599 | 0.011304 | 0.009468 | 0.018174 | ... | -0.011304 | 0.012201 | -0.018174 | 0.000000 | 0.009562 | 0.045314 | 0.004301 | 0.016178 | 0.000878 | 0.000878 |
| 1 | 1.786845 | 1290.151507 | True | 0.0 | 0.0 | 0.014591 | 0.110154 | 0.009917 | 0.002832 | 0.025619 | ... | -0.009917 | 0.011198 | -0.025619 | 0.000000 | 0.004122 | 0.061147 | 0.016209 | 0.017420 | 0.000090 | 0.000090 |
| 2 | 1.171520 | 1255.933868 | False | 0.0 | 0.0 | 0.080784 | 0.193537 | 0.046615 | 0.050574 | 0.077199 | ... | 0.046615 | 0.014686 | -0.077199 | 0.000000 | 0.003245 | 0.031201 | 0.040466 | 0.018319 | 0.000234 | 0.000234 |
| 3 | 2.143416 | 1292.669093 | False | 0.0 | 0.0 | 0.012502 | 0.119081 | 0.015465 | 0.006444 | 0.033388 | ... | -0.015465 | 0.012439 | -0.033388 | 0.000000 | 0.003909 | 0.054443 | 0.013245 | 0.019323 | 0.002988 | 0.002988 |
| 4 | 2.763538 | 1243.469600 | False | 0.0 | 0.0 | 0.002154 | 0.115101 | 0.025085 | 0.033393 | 0.033542 | ... | -0.025085 | 0.009568 | -0.033542 | 0.000000 | 0.001315 | 0.054153 | 0.018201 | 0.027943 | 0.005728 | 0.005728 |
| 5 | 0.535148 | 1328.353392 | False | 0.0 | 0.0 | 0.030307 | 0.151008 | 0.003629 | 0.013160 | 0.011592 | ... | -0.003629 | 0.011592 | 0.011592 | 0.297720 | 0.277125 | 0.060562 | 0.023733 | 0.019456 | 0.006112 | 0.006112 |
| 6 | 0.493360 | 1347.611001 | True | 0.0 | 0.0 | 0.005393 | 0.109769 | 0.003040 | 0.009391 | 0.013188 | ... | -0.003040 | 0.013188 | 0.013188 | 0.130559 | 0.110135 | 0.055566 | 0.030226 | 0.015652 | 0.002710 | 0.002710 |
| 7 | 6.377576 | 1377.397773 | False | 0.0 | 0.0 | 0.039499 | 0.089259 | 0.019500 | 0.013119 | 0.014794 | ... | -0.019500 | 0.014794 | 0.014794 | 0.148010 | 0.125617 | 0.046990 | 0.027492 | 0.018277 | 0.011835 | 0.011835 |
| 8 | 5.742168 | 1390.374437 | False | 0.0 | 0.0 | 0.033597 | 0.097318 | 0.031008 | 0.032361 | 0.006409 | ... | -0.031008 | 0.015508 | 0.006409 | 0.001101 | 0.017485 | 0.054544 | 0.014511 | 0.015824 | 0.008619 | 0.008619 |
| 9 | 1.396263 | 1339.752950 | True | 0.0 | 0.0 | 0.000606 | 0.131231 | 0.013266 | 0.018402 | 0.011957 | ... | -0.013266 | 0.011957 | 0.011957 | 0.003516 | 0.010965 | 0.062936 | 0.011776 | 0.018199 | 0.004581 | 0.004581 |
| 10 | 0.644651 | 1342.785453 | False | 0.0 | 0.0 | 0.032515 | 0.157280 | 0.000830 | 0.003555 | 0.013284 | ... | 0.000830 | 0.013284 | 0.013284 | 0.857156 | 0.836370 | 0.045922 | 0.039329 | 0.016460 | 0.003218 | 0.003218 |
| 11 | -1.883972 | 1312.045437 | False | 0.0 | 0.0 | 0.006324 | 0.129135 | 0.000306 | 0.002289 | 0.013585 | ... | -0.000306 | 0.013585 | 0.013585 | 0.001648 | 0.014651 | 0.051055 | 0.029518 | 0.017340 | 0.009204 | 0.009204 |
| 12 | 1.211110 | 1344.398677 | True | 0.0 | 0.0 | 0.027407 | 0.081454 | 0.015105 | 0.018898 | 0.013175 | ... | -0.015105 | 0.013175 | 0.013175 | 0.574581 | 0.555774 | 0.054522 | 0.033541 | 0.016879 | 0.002401 | 0.002401 |
| 13 | -14.685454 | 1217.300913 | False | 0.0 | 0.0 | 0.046407 | 0.162372 | 0.017760 | 0.005585 | 0.009986 | ... | 0.017760 | 0.009986 | 0.009986 | 0.052732 | 0.042651 | 0.060200 | 0.020443 | 0.018975 | 0.018250 | 0.018250 |
| 14 | -14.685454 | 1217.300913 | False | 0.0 | 0.0 | 0.046407 | 0.162372 | 0.017760 | 0.005585 | 0.009986 | ... | 0.017760 | 0.009986 | 0.009986 | 0.052732 | 0.042651 | 0.060200 | 0.020443 | 0.018975 | 0.018250 | 0.018250 |
| 15 | -14.685454 | 1217.300913 | False | 0.0 | 0.0 | 0.046407 | 0.162372 | 0.017760 | 0.005585 | 0.009986 | ... | 0.017760 | 0.009986 | 0.009986 | 0.052732 | 0.042651 | 0.060200 | 0.020443 | 0.018975 | 0.018250 | 0.018250 |
| 16 | 2.168816 | 1352.629530 | True | 0.0 | 0.0 | 0.014033 | 0.127948 | 0.012246 | 0.016146 | 0.014315 | ... | -0.012246 | 0.014315 | 0.014315 | 0.356916 | 0.342040 | 0.055025 | 0.032680 | 0.018283 | 0.002118 | 0.002118 |
| 17 | NaN | NaN | False | NaN | NaN | NaN | NaN | NaN | NaN | NaN | ... | NaN | NaN | NaN | 0.603117 | 0.588110 | NaN | NaN | NaN | NaN | NaN |
| 18 | 1.315338 | 1342.522331 | True | 0.0 | 0.0 | 0.012280 | 0.126901 | 0.010194 | 0.015470 | 0.013084 | ... | -0.010194 | 0.013084 | 0.013084 | 0.160617 | 0.141226 | 0.063446 | 0.018274 | 0.017580 | 0.003858 | 0.003858 |
| 19 | 3.494361 | 1370.176239 | True | 0.0 | 0.0 | 0.007055 | 0.136635 | 0.015152 | 0.023994 | 0.013518 | ... | -0.015152 | 0.013518 | 0.013518 | 0.310037 | 0.296888 | 0.059237 | 0.027832 | 0.016558 | 0.002479 | 0.002479 |
20 rows × 132 columns
Example 3 - Cpx-only Barometry
Very similar to above, just don’t need liq_comps input
3a -Pressure only, using equation 32b (at T=1300 K), and H2O=0
This equation requires H2O content in the liquid. If you don’t enter anything, it assumes H2O=0
else specify using H2O_Liq=….
it prints a warning telling you that by defualt, this is what the function is doing
[16]:
eq32b_noH=pt.calculate_cpx_only_press(cpx_comps=Cpxs, T=1300,
equationP="P_Put2008_eq32b")
eq32b_noH
c:\users\penny\box\postdoc\mybarometers\thermobar_outer\src\Thermobar\clinopyroxene_thermobarometry.py:3293: UserWarning: This Cpx-only barometer is sensitive to H2O content of the liquid. By default, this function uses H2O=0 wt%, else you can enter a value of H2O_Liq in the function
w.warn('This Cpx-only barometer is sensitive to H2O content of the liquid. '
[16]:
0 -1.567381
1 -1.202221
2 -0.317946
3 -0.996608
4 0.467437
5 -6.911370
6 -6.286860
7 -11.586143
8 -3.498491
9 -13.658623
10 -11.540359
11 -13.391546
12 -14.272181
13 -18.202136
14 -18.202136
15 -18.202136
16 -10.918332
17 NaN
18 -5.618846
19 -8.241059
dtype: float64
3b - Pressure only, using 5 wt% water
[17]:
eq32b_5H=pt.calculate_cpx_only_press(cpx_comps=Cpxs, T=1300,
equationP="P_Put2008_eq32b", H2O_Liq=5)
eq32b_5H
[17]:
0 0.697619
1 1.062779
2 1.947054
3 1.268392
4 2.732437
5 -4.646370
6 -4.021860
7 -9.321143
8 -1.233491
9 -11.393623
10 -9.275359
11 -11.126546
12 -12.007181
13 -15.937136
14 -15.937136
15 -15.937136
16 -8.653332
17 NaN
18 -3.353846
19 -5.976059
dtype: float64
3c - Temperature-only using eq 32d at 5 kbar
[18]:
eq32d_5kbar=pt.calculate_cpx_only_temp(cpx_comps=Cpxs, equationT="T_Put2008_eq32d",
P=5)
eq32d_5kbar
[18]:
0 1457.849197
1 1441.107847
2 1415.899813
3 1455.722443
4 1441.512524
5 1413.510042
6 1420.741893
7 1406.196227
8 1463.728870
9 1393.605045
10 1239.894412
11 1413.752159
12 1402.402180
13 1360.089648
14 1360.089648
15 1360.089648
16 1366.264788
17 1381.658652
18 1396.089868
19 1412.298889
dtype: float64
3d - Iterating P from 32b, and T from 32d, with H2O=5
[19]:
eq32b_32d_5H=pt.calculate_cpx_only_press_temp(cpx_comps=Cpxs, equationT="T_Put2008_eq32d",
equationP="P_Put2008_eq32b", H2O_Liq=5)
eq32b_32d_5H
[19]:
| P_kbar_calc | T_K_calc | Delta_P_kbar_Iter | Delta_T_K_Iter | SiO2_Cpx | TiO2_Cpx | Al2O3_Cpx | FeOt_Cpx | MnO_Cpx | MgO_Cpx | ... | Jd | Jd_from 0=Na, 1=Al | CaTs | CaTi | DiHd_1996 | EnFs | DiHd_2003 | Di_Cpx | FeIII_Wang21 | FeII_Wang21 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3.889323 | 1448.656494 | 0.000000e+00 | 0.000000e+00 | 51.500000 | 0.500000 | 3.700000 | 5.180000 | 0.090000 | 15.800000 | ... | 0.017055 | 0 | 0.030375 | 0.041013 | 0.814436 | 0.103827 | 0.814436 | 0.686042 | 0.035332 | 0.123446 |
| 1 | 3.721484 | 1430.647515 | 0.000000e+00 | 0.000000e+00 | 50.300000 | 0.730000 | 4.120000 | 5.830000 | 0.000000 | 15.000000 | ... | 0.017330 | 0 | 0.036817 | 0.044938 | 0.819939 | 0.097220 | 0.819939 | 0.673165 | 0.040732 | 0.140847 |
| 2 | 3.982623 | 1407.721620 | 0.000000e+00 | 0.000000e+00 | 47.300000 | 1.750000 | 7.850000 | 6.510000 | 0.140000 | 13.100000 | ... | 0.018085 | 0 | 0.091885 | 0.071667 | 0.732671 | 0.099545 | 0.732671 | 0.570239 | 0.038619 | 0.164508 |
| 3 | 4.567865 | 1452.151021 | 2.273737e-13 | 1.818989e-12 | 51.100000 | 0.630000 | 4.410000 | 5.660000 | 0.130000 | 15.600000 | ... | 0.016335 | 0 | 0.045827 | 0.041197 | 0.796049 | 0.114596 | 0.796049 | 0.658834 | 0.039859 | 0.133524 |
| 4 | 5.941303 | 1449.216073 | 0.000000e+00 | 0.000000e+00 | 51.000000 | 0.560000 | 4.140000 | 7.330000 | 0.200000 | 14.400000 | ... | 0.022215 | 0 | 0.043110 | 0.035953 | 0.806694 | 0.106652 | 0.806694 | 0.623678 | 0.038138 | 0.188429 |
| 5 | -4.055503 | 1340.840204 | 2.273737e-13 | 1.591616e-12 | 51.893554 | 0.985648 | 4.374039 | 6.014774 | 0.427037 | 15.882719 | ... | 0.013343 | 1 | 0.000000 | 0.084295 | 0.792618 | 0.110258 | 0.792618 | 0.645602 | 0.105585 | 0.071935 |
| 6 | -3.177367 | 1354.783292 | 0.000000e+00 | 0.000000e+00 | 51.671503 | 1.222254 | 4.424609 | 5.755014 | 0.537483 | 15.849673 | ... | 0.012942 | 1 | 0.000000 | 0.085792 | 0.788624 | 0.108885 | 0.788624 | 0.644849 | 0.091360 | 0.078945 |
| 7 | -9.419020 | 1291.083222 | 0.000000e+00 | 0.000000e+00 | 52.462671 | 0.861366 | 4.306456 | 5.521574 | 0.093405 | 15.931721 | ... | 0.030112 | 1 | 0.000000 | 0.074483 | 0.768475 | 0.116216 | 0.768475 | 0.641596 | 0.085759 | 0.077164 |
| 8 | 1.553018 | 1435.084291 | 0.000000e+00 | 0.000000e+00 | 52.341465 | 0.558521 | 4.332519 | 5.307290 | 0.531408 | 16.743678 | ... | 0.024443 | 0 | 0.009099 | 0.069054 | 0.768797 | 0.135942 | 0.768797 | 0.642889 | 0.071195 | 0.085917 |
| 9 | -11.734771 | 1261.200708 | -2.273737e-13 | -1.591616e-12 | 51.905733 | 0.643097 | 3.880163 | 5.651830 | 0.614606 | 16.146415 | ... | 0.013619 | 1 | 0.000000 | 0.074712 | 0.802701 | 0.111995 | 0.802701 | 0.659037 | 0.116120 | 0.052394 |
| 10 | -9.147761 | 1140.304548 | 0.000000e+00 | 0.000000e+00 | 51.705545 | 0.697444 | 4.406196 | 5.746137 | 0.443832 | 15.907585 | ... | 0.013242 | 1 | 0.000000 | 0.085252 | 0.784209 | 0.112455 | 0.784209 | 0.643591 | 0.159222 | 0.010809 |
| 11 | -11.304233 | 1282.889304 | 0.000000e+00 | 0.000000e+00 | 52.200291 | 1.467759 | 4.077400 | 5.607369 | 0.832626 | 16.045590 | ... | 0.008136 | 1 | 0.000000 | 0.080574 | 0.778118 | 0.114853 | 0.778118 | 0.634926 | 0.082786 | 0.082406 |
| 12 | -12.328411 | 1264.435549 | 0.000000e+00 | 0.000000e+00 | 51.725315 | 0.745761 | 4.586485 | 5.736715 | 0.550561 | 16.407623 | ... | 0.014478 | 1 | 0.000000 | 0.088062 | 0.774008 | 0.128816 | 0.774008 | 0.636936 | 0.130621 | 0.038544 |
| 13 | -16.327464 | 1195.406373 | 0.000000e+00 | 0.000000e+00 | 51.892711 | 0.918335 | 3.879493 | 6.001785 | 0.224476 | 16.766705 | ... | 0.000724 | 1 | 0.000000 | 0.080643 | 0.789095 | 0.137203 | 0.789095 | 0.653001 | 0.167476 | 0.010370 |
| 14 | -16.327464 | 1195.406373 | 0.000000e+00 | 0.000000e+00 | 51.892711 | 0.918335 | 3.879493 | 6.001785 | 0.224476 | 16.766705 | ... | 0.000724 | 1 | 0.000000 | 0.080643 | 0.789095 | 0.137203 | 0.789095 | 0.653001 | 0.167476 | 0.010370 |
| 15 | -16.327464 | 1195.406373 | 0.000000e+00 | 0.000000e+00 | 51.892711 | 0.918335 | 3.879493 | 6.001785 | 0.224476 | 16.766705 | ... | 0.000724 | 1 | 0.000000 | 0.080643 | 0.789095 | 0.137203 | 0.789095 | 0.653001 | 0.167476 | 0.010370 |
| 16 | -9.015029 | 1257.554261 | 0.000000e+00 | 0.000000e+00 | 52.006582 | 1.490563 | 4.633149 | 6.150716 | 0.383969 | 15.823193 | ... | 0.016166 | 1 | 0.000000 | 0.087705 | 0.765280 | 0.121381 | 0.765280 | 0.621245 | 0.130527 | 0.049936 |
| 17 | NaN | NaN | NaN | NaN | 51.986059 | 1.119548 | 4.107258 | 5.587534 | 0.869447 | 16.661904 | ... | 0.000000 | 1 | 0.000000 | 0.087680 | 0.785434 | 0.125194 | 0.785434 | 0.644977 | 0.159251 | 0.004758 |
| 18 | -2.884479 | 1333.597198 | 0.000000e+00 | 0.000000e+00 | 52.066983 | 1.070126 | 4.261572 | 5.845278 | 0.276057 | 16.038723 | ... | 0.013722 | 1 | 0.000000 | 0.081720 | 0.779896 | 0.117953 | 0.779896 | 0.642299 | 0.131180 | 0.041246 |
| 19 | -5.607345 | 1327.248553 | 2.273737e-13 | 1.818989e-12 | 51.973899 | 0.809677 | 4.665615 | 5.864718 | 0.840250 | 16.467894 | ... | 0.019037 | 1 | 0.000000 | 0.087068 | 0.777534 | 0.128604 | 0.777534 | 0.632774 | 0.145446 | 0.026854 |
20 rows × 49 columns
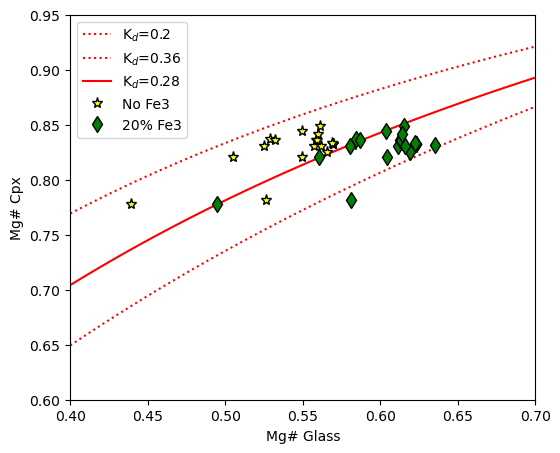
Example 4 - Plotting a Cpx-Liq Rhodes diagram to assess Fe-Mg equilibrium using fixed Kd values
The function calculate_cpx_rhodes_diagram_lines calculates the lines needed for the plot in a number of ways
There is disagrement in the literature as to whether Kd Fe-Mg should be assessed using just Fe2+ in the melt, or FeT, so we show both scenarios here.
Step 1 - Calculate Mg# for liq and cpxs
A number of functions in thermobar let you do it, we use this one here because it returns Mg#s for both phases
[20]:
cpx_comps_Fe3=pt.calculate_clinopyroxene_liquid_components(liq_comps=Liqs,
cpx_comps=Cpxs, Fe3Fet_Liq=0.2)
Mgnos of Cpx are stored in the column accesed by cpx_comps_Fe3[‘Mgno_Cpx’]
Mgnos of Liq are stored in the column accesed by cpx_comps_Fe3[‘Mgno_Liq_noFe3’], for no Fe3, or cpx_comps_Fe3[‘Mgno_Liq_Fe2’] using just Fe2+ (e.g., 20% of Fe is 3+ here)
Step 2 - Calculate equilibrium lines to show on the rhodes diagram
You tell the function the min and max glass Mg# you want to show, e.g., the xspan of your plot
It returns lines for Kd=0.28+-0.08 after Putirka (2008)
[21]:
# Want to calculate Mg# to show on diagram between say 0.4 and 0.7 for the glass
eq_lines_1=pt.calculate_cpx_rhodes_diagram_lines(Min_Mgno=0.4, Max_Mgno=0.7)
eq_lines_1
[21]:
| Mg#_Liq | Eq_Cpx_Mg# (Kd=0.28) | Eq_Cpx_Mg# (Kd=0.2) | Eq_Cpx_Mg# (Kd=0.36) | |
|---|---|---|---|---|
| 0 | 0.400000 | 0.704225 | 0.769231 | 0.649351 |
| 1 | 0.403030 | 0.706845 | 0.771462 | 0.652217 |
| 2 | 0.406061 | 0.709445 | 0.773672 | 0.655065 |
| 3 | 0.409091 | 0.712025 | 0.775862 | 0.657895 |
| 4 | 0.412121 | 0.714586 | 0.778032 | 0.660707 |
| ... | ... | ... | ... | ... |
| 95 | 0.687879 | 0.887273 | 0.916801 | 0.859588 |
| 96 | 0.690909 | 0.888681 | 0.917874 | 0.861287 |
| 97 | 0.693939 | 0.890081 | 0.918941 | 0.862979 |
| 98 | 0.696970 | 0.891473 | 0.920000 | 0.864662 |
| 99 | 0.700000 | 0.892857 | 0.921053 | 0.866337 |
100 rows × 4 columns
Step 3 - Combine these on a plot
You might need to adust the x and y limits of this plot for your own data
[22]:
fig, (ax1) = plt.subplots(1, 1, figsize = (6,5))
ax1.plot(eq_lines_1['Mg#_Liq'], eq_lines_1['Eq_Cpx_Mg# (Kd=0.2)'], ':r', label="K$_d$=0.2")
ax1.plot(eq_lines_1['Mg#_Liq'], eq_lines_1['Eq_Cpx_Mg# (Kd=0.36)'], ':r', label="K$_d$=0.36")
ax1.plot(eq_lines_1['Mg#_Liq'], eq_lines_1['Eq_Cpx_Mg# (Kd=0.28)'], '-r', label="K$_d$=0.28")
ax1.plot(cpx_comps_Fe3['Mgno_Liq_noFe3'], cpx_comps_Fe3['Mgno_Cpx'], '*k', mfc='yellow', ms=8, label="No Fe3")
ax1.plot(cpx_comps_Fe3['Mgno_Liq_Fe2'], cpx_comps_Fe3['Mgno_Cpx'], 'dk', mfc='green', ms=8, label="20% Fe3")
ax1.legend()
ax1.set_xlabel('Mg# Glass')
ax1.set_ylabel('Mg# Cpx')
# adjust x and y limits
ax1.set_xlim([0.4, 0.7])
ax1.set_ylim([0.6, 0.95])
[22]:
(0.6, 0.95)
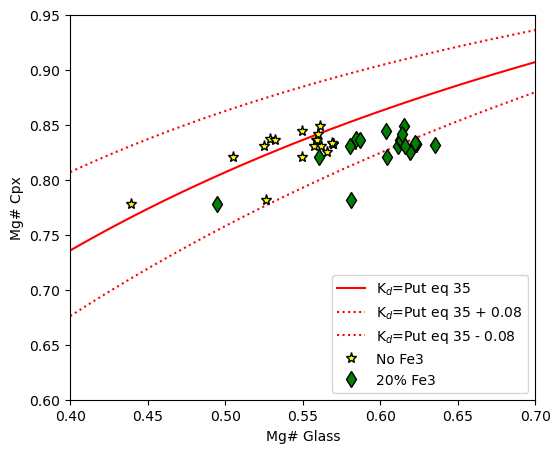
Example 5 - Rhodes diagram using equation 35 of Putirka to calculate Kd as a function of T.
Here, we plot the equilibrium fields on the Rhodes diagram using equation 35 of Putirka, which is T-sensitive
Must specify T in Kelvin. Then return column for default 0.28, as well as the results for Putirka eq 35.
[23]:
eq_lines_2=pt.calculate_cpx_rhodes_diagram_lines(Min_Mgno=0.4, Max_Mgno=0.7, T=1300)
eq_lines_2.head()
[23]:
| Mg#_Liq | Eq_Cpx_Mg# (Kd=0.28) | Eq_Cpx_Mg# (Kd=0.2) | Eq_Cpx_Mg# (Kd=0.36) | Kd_Eq35_P2008 | Eq_Cpx_Mg# (Kd from Eq 35 P2008) | Eq_Cpx_Mg# (Eq 35 P2008)+0.08 | Eq_Cpx_Mg# (Eq 35 P2008)-0.08 | |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.400000 | 0.704225 | 0.769231 | 0.649351 | 0.239475 | 0.735720 | 0.676036 | 0.806964 |
| 1 | 0.403030 | 0.706845 | 0.771462 | 0.652217 | 0.239475 | 0.738165 | 0.678791 | 0.808921 |
| 2 | 0.406061 | 0.709445 | 0.773672 | 0.655065 | 0.239475 | 0.740589 | 0.681528 | 0.810858 |
| 3 | 0.409091 | 0.712025 | 0.775862 | 0.657895 | 0.239475 | 0.742993 | 0.684246 | 0.812775 |
| 4 | 0.412121 | 0.714586 | 0.778032 | 0.660707 | 0.239475 | 0.745377 | 0.686945 | 0.814673 |
[24]:
fig, (ax1) = plt.subplots(1, 1, figsize = (6,5))
ax1.plot(eq_lines_2['Mg#_Liq'], eq_lines_2['Eq_Cpx_Mg# (Kd from Eq 35 P2008)'], '-r', label="K$_d$=Put eq 35")
ax1.plot(eq_lines_2['Mg#_Liq'], eq_lines_2['Eq_Cpx_Mg# (Eq 35 P2008)+0.08'], ':r', label="K$_d$=Put eq 35 + 0.08")
ax1.plot(eq_lines_2['Mg#_Liq'], eq_lines_2['Eq_Cpx_Mg# (Eq 35 P2008)-0.08'], ':r', label="K$_d$=Put eq 35 - 0.08")
ax1.set_xlim([0.4, 0.7])
ax1.plot(cpx_comps_Fe3['Mgno_Liq_noFe3'], cpx_comps_Fe3['Mgno_Cpx'], '*k', mfc='yellow', ms=8, label="No Fe3")
ax1.plot(cpx_comps_Fe3['Mgno_Liq_Fe2'], cpx_comps_Fe3['Mgno_Cpx'], 'dk', mfc='green', ms=8, label="20% Fe3")
ax1.legend()
ax1.set_xlabel('Mg# Glass')
ax1.set_ylabel('Mg# Cpx')
# adjust x and y limits
ax1.set_xlim([0.4, 0.7])
ax1.set_ylim([0.6, 0.95])
[24]:
(0.6, 0.95)
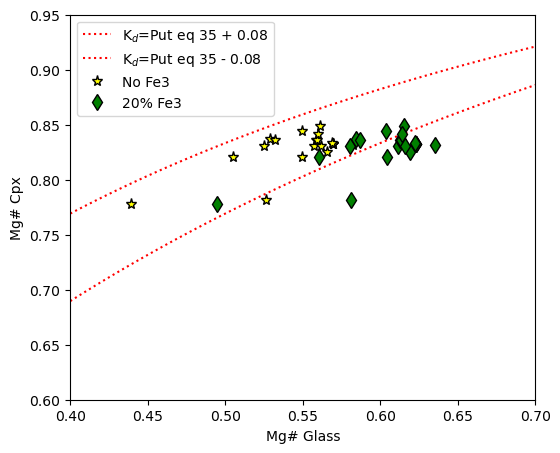
Example 6 - You can also specify a minimum and maximum Kd value you wish to calculate Rhodes lines for (here 0.2, 0.3)
[25]:
eq_lines_3=pt.calculate_cpx_rhodes_diagram_lines(Min_Mgno=0.4, Max_Mgno=0.7, KdMin=0.2, KdMax=0.3)
eq_lines_3.head()
[25]:
| Mg#_Liq | Eq_Cpx_Mg# (Kd=0.28) | Eq_Cpx_Mg# (Kd=0.2) | Eq_Cpx_Mg# (Kd=0.36) | Eq_Cpx_Mg# (KdMin=0.2) | Eq_Cpx_Mg# (KdMax=0.3) | |
|---|---|---|---|---|---|---|
| 0 | 0.400000 | 0.704225 | 0.769231 | 0.649351 | 0.769231 | 0.689655 |
| 1 | 0.403030 | 0.706845 | 0.771462 | 0.652217 | 0.771462 | 0.692348 |
| 2 | 0.406061 | 0.709445 | 0.773672 | 0.655065 | 0.773672 | 0.695021 |
| 3 | 0.409091 | 0.712025 | 0.775862 | 0.657895 | 0.775862 | 0.697674 |
| 4 | 0.412121 | 0.714586 | 0.778032 | 0.660707 | 0.778032 | 0.700309 |
[26]:
fig, (ax1) = plt.subplots(1, 1, figsize = (6,5))
ax1.plot(eq_lines_3['Mg#_Liq'], eq_lines_3['Eq_Cpx_Mg# (KdMin=0.2)'], ':r', label="K$_d$=Put eq 35 + 0.08")
ax1.plot(eq_lines_3['Mg#_Liq'], eq_lines_3['Eq_Cpx_Mg# (KdMax=0.3)'], ':r', label="K$_d$=Put eq 35 - 0.08")
ax1.set_xlim([0.4, 0.7])
ax1.plot(cpx_comps_Fe3['Mgno_Liq_noFe3'], cpx_comps_Fe3['Mgno_Cpx'], '*k', mfc='yellow', ms=8, label="No Fe3")
ax1.plot(cpx_comps_Fe3['Mgno_Liq_Fe2'], cpx_comps_Fe3['Mgno_Cpx'], 'dk', mfc='green', ms=8, label="20% Fe3")
ax1.legend()
ax1.set_xlabel('Mg# Glass')
ax1.set_ylabel('Mg# Cpx')
# adjust x and y limits
ax1.set_xlim([0.4, 0.7])
ax1.set_ylim([0.6, 0.95])
[26]:
(0.6, 0.95)
Example 7 - Can get all options by specifying a temp, and a min and max Kd
Can then plot them however you want.
[27]:
eq_lines_4=pt.calculate_cpx_rhodes_diagram_lines(Min_Mgno=0.4, Max_Mgno=0.7, T=1300, KdMin=0.2, KdMax=0.3)
eq_lines_4.head()
[27]:
| Mg#_Liq | Eq_Cpx_Mg# (Kd=0.28) | Eq_Cpx_Mg# (Kd=0.2) | Eq_Cpx_Mg# (Kd=0.36) | Kd_Eq35_P2008 | Eq_Cpx_Mg# (Kd from Eq 35 P2008) | Eq_Cpx_Mg# (Eq 35 P2008)+0.08 | Eq_Cpx_Mg# (Eq 35 P2008)-0.08 | Eq_Cpx_Mg# (KdMin=0.2) | Eq_Cpx_Mg# (KdMax=0.3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.400000 | 0.704225 | 0.769231 | 0.649351 | 0.239475 | 0.735720 | 0.676036 | 0.806964 | 0.769231 | 0.689655 |
| 1 | 0.403030 | 0.706845 | 0.771462 | 0.652217 | 0.239475 | 0.738165 | 0.678791 | 0.808921 | 0.771462 | 0.692348 |
| 2 | 0.406061 | 0.709445 | 0.773672 | 0.655065 | 0.239475 | 0.740589 | 0.681528 | 0.810858 | 0.773672 | 0.695021 |
| 3 | 0.409091 | 0.712025 | 0.775862 | 0.657895 | 0.239475 | 0.742993 | 0.684246 | 0.812775 | 0.775862 | 0.697674 |
| 4 | 0.412121 | 0.714586 | 0.778032 | 0.660707 | 0.239475 | 0.745377 | 0.686945 | 0.814673 | 0.778032 | 0.700309 |
Calcs with all possible equations
[28]:
# If you want to run calcs with the machine learning expressions,
# you need to install this extra package. Else, this will work fine without and just not give those answers
[8]:
# uncomment this line if you havent installed already!
#!pip install "https://github.com/PennyWieser/Thermobar_onnx/archive/refs/tags/v.0.0.4.zip"
[9]:
all_pt_cpx_liq=pt.calculate_cpx_liq_press_all_eqs(liq_comps=Liqs,
cpx_comps=Cpxs)
all_pt_cpx_liq.head()
We have reset the index on Cpx and Liq comps for the Petrelli expressions
Youve selected a P-independent function
Youve selected a T-independent function
Youve selected a T-independent function
Im normalizing using the Jorgenson method, e.g. 100 total, 2dp
Im normalizing using the Jorgenson method, e.g. 100 total, 2dp
Youve selected a P-independent function
Youve selected a T-independent function
Youve selected a T-independent function
[9]:
| P_kbar: (P_Neave17, T_Put2008_eq33) | T_K: (P_Neave17, T_Put2008_eq33) | P_kbar: (P_Neave17, T_Put2008_eq34_cpx_sat) | T_K: (P_Neave17, T_Put2008_eq34_cpx_sat) | P_kbar: (P_Put2008_eq30, T_Put2008_eq33) | T_K: (P_Put2008_eq30, T_Put2008_eq33) | P_kbar: (P_Put2008_eq31, T_Put2008_eq33) | T_K: (P_Put2008_eq31, T_Put2008_eq33) | P_kbar: (P_Put2008_eq32c, T_Put2008_eq33) | T_K: (P_Put2008_eq32c, T_Put2008_eq33) | ... | P_kbar: (P_Put2008_eq32c, T_Put2008_eq34) | T_K: (P_Put2008_eq32c, T_Put2008_eq34)) | P_kbar: (P2003 P&T) | T_K: (P2003 P&T) | T_K: (P_Put1996_eqP1, T_Put1996_eqT2) | P_kbar: (P_Put1996_eqP1, T_Put1996_eqT2) | P_kbar: (Petrelli, 2020) | T_K: (Petrelli, 2020) | P_kbar: (Jorgenson, 2022) | T_K: (Jorgenson, 2022) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.960631 | 1344.698427 | 1.161345 | 1372.216226 | 2.530914 | 1352.408784 | 2.386186 | 1351.694450 | 2.209868 | 1350.825212 | ... | 4.653251 | 1402.264846 | 0.947885 | 1429.684711 | 1431.212441 | 2.587626 | 2.329420 | 1365.986364 | 1.0 | 1355.15 |
| 1 | 1.583720 | 1289.239285 | 1.816406 | 1323.941135 | 1.786845 | 1290.151507 | 2.624094 | 1293.925217 | 1.870946 | 1290.529579 | ... | 4.453012 | 1344.888862 | 0.453851 | 1388.379217 | 1410.593746 | 1.759554 | 2.445954 | 1282.522727 | 2.0 | 1285.15 |
| 2 | 0.895589 | 1254.759806 | 1.055286 | 1283.601656 | 1.171520 | 1255.933868 | 5.162310 | 1273.163405 | 6.281137 | 1278.078913 | ... | 9.615150 | 1348.268665 | -0.946284 | 1362.151670 | 1402.050070 | 0.278452 | 2.000000 | 1238.150000 | 2.0 | 1238.15 |
| 3 | 0.952699 | 1287.319189 | 1.130722 | 1317.160073 | 2.143416 | 1292.669093 | 2.225915 | 1293.041408 | 2.332966 | 1293.524848 | ... | 4.859417 | 1346.713288 | -0.021555 | 1387.134284 | 1408.805156 | 1.263705 | 2.000000 | 1273.150000 | 2.0 | 1273.15 |
| 4 | 3.581562 | 1246.894077 | 3.771286 | 1283.856794 | 2.763538 | 1243.469600 | 2.113770 | 1240.762861 | 1.456253 | 1238.035814 | ... | 3.559098 | 1282.306231 | -1.003005 | 1307.655434 | 1373.636951 | 1.791782 | 2.000000 | 1238.150000 | 2.0 | 1238.15 |
5 rows × 26 columns
[13]:
all_pt_cpx_only=pt.calculate_cpx_only_press_all_eqs(
cpx_comps=Cpxs)
all_pt_cpx_only.head()
[13]:
| P_Wang21_eq1 | T_Wang21_eq2 | T_Jorgenson22 | P_Jorgenson22 | T_Petrelli20 | T_Put_Teq32d_Peq32a | T_Put_Teq32d_Peq32b | P_Petrelli20 | P_Put_Teq32d_Peq32a | P_Put_Teq32d_Peq32b | ... | EnFs | DiHd_2003 | Di_Cpx | FeIII_Wang21 | FeII_Wang21 | H2O_Liq | T_Put_Teq32d_subsol_Peq32a | T_Put_Teq32d_subsol_Peq32b | P_Put_Teq32d_subsol_Peq32a | P_Put_Teq32d_subsol_Peq32b | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1.171230 | 1440.358697 | 1355.152954 | 1.000001 | 1342.737793 | 1429.010995 | 1423.934424 | 2.002911 | 1.515722 | 0.902362 | ... | 0.103827 | 0.814436 | 0.686042 | 0.035332 | 0.123446 | None | 1147.861295 | 1083.173193 | 7.038781 | -0.307139 |
| 1 | 0.500630 | 1429.224347 | 1285.151611 | 2.000002 | 1332.734375 | 1413.354443 | 1406.830074 | 2.376496 | 1.607834 | 0.810392 | ... | 0.097220 | 0.819939 | 0.673165 | 0.040732 | 0.140847 | None | 1126.101733 | 1056.468462 | 8.994531 | 0.813312 |
| 2 | 0.882985 | 1373.014871 | 1238.150146 | 2.000002 | 1238.154541 | 1416.604337 | 1385.069856 | 2.000006 | 5.087644 | 1.164715 | ... | 0.099545 | 0.732671 | 0.570239 | 0.038619 | 0.164508 | None | 1131.385058 | 1047.494247 | 12.020262 | 1.985596 |
| 3 | 1.452333 | 1433.283620 | 1273.152954 | 2.000002 | 1273.150513 | 1436.877879 | 1427.367498 | 2.000006 | 2.719843 | 1.569105 | ... | 0.114596 | 0.796049 | 0.658834 | 0.039859 | 0.133524 | None | 1164.764522 | 1100.925908 | 6.984321 | -0.156942 |
| 4 | 1.986290 | 1418.418850 | 1238.150146 | 2.000002 | 1238.154541 | 1427.089374 | 1424.835650 | 2.000006 | 3.237622 | 2.962238 | ... | 0.106652 | 0.806694 | 0.623678 | 0.038138 | 0.188429 | None | 1155.385488 | 1095.094118 | 8.306445 | 1.438251 |
5 rows × 60 columns